Для лучшей сохранности продуктов важно знать правильные условия их хранения. Часто для этих целей
Полезные рекомендации о том, как правильно подобрать сыр, посуду и преподнести блюдо, следующие:
Исландский мох от кашля является растительным и достаточно эффективным средством, которое позволяет бороться с
Как приготовить невероятные сырные палочки к пиву. Пятерка лучших рецепта на все случаи жизни
Производство пива у российских предпринимателей пользуется особой популярностью. Такой бизнес востребован, в том числе
Представляем рецепты приготовления венского пива в домашних условиях, в лучших традициях европейских пивоваров. Два
Джош Вайкерт делится на сайте Beer&Brewing рецептом приготовления качественного вайсбира. Pivo.by публикует перевод материала.
Вайсбир (Weissbier) - это немецкое пшеничное пиво, также называемое как Weiss, которое варится с
Описание пива Stout (Стаут). История возникновения бренда. Дегустационные особенности напитка. Характеристика и виды пива
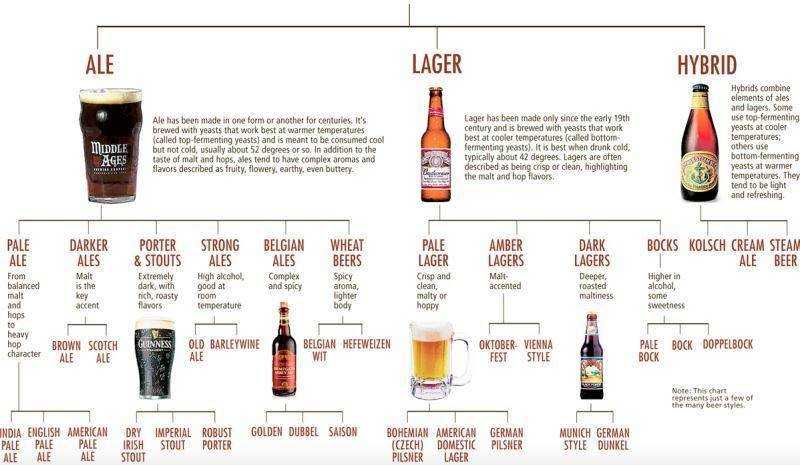
Описание основных направлений в современном пивоварении. Различия между стилями пива.
Грюйт, сахти, можжевеловое пиво и вересковый эль еще недавно казались почти вымершей экзотикой. Но
Классический немецкий янтарный лагер с копчёным солодом можно легко сварить дома.
Тёмное пиво с добавлением овса когда-то позиционировали как укрепляющий здоровье напиток.
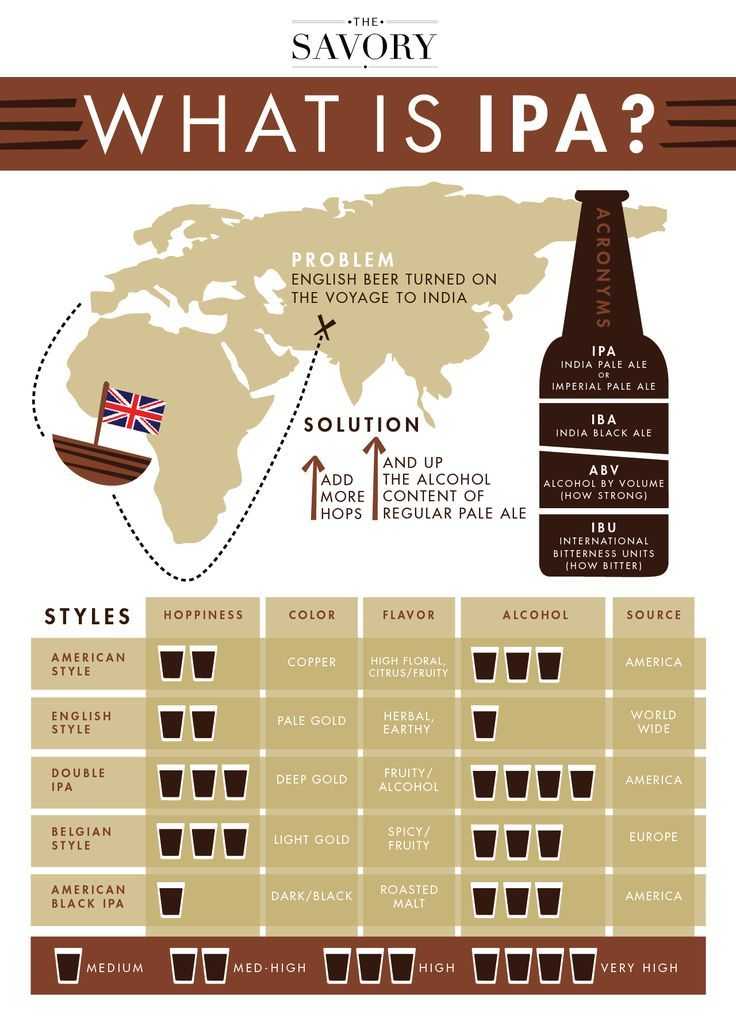
По определению, India pale ale - это на самом деле сильно охмеленное светлое пиво,
Пиво ИПА – класс необычно горьких, но при этом мягких и запоминающихся продуктов, которые
Пилснер — это разновидность лагера, родом из Чешской Республики, отличительными чертами которого являются выраженная
Немецкие стили пива: пилснер и хеллес, майбок и традиционный бок, доппельбок и айсбок, октоберфест,
В России же, как известно, в почете был и остается другой напиток самостоятельного изготовления,
Автор блога Hors Catégorie Brewing Дэйв Янссен посвятил одну из статей исследованию показателей горечи
Немецкие бюргеры давно поняли, какую прибыль приносят карнавалы, и вовсю ими пользуются.
Читайте в блоге Winestyle.ru статью «Пиво стаут». Свежие статьи для гурманов и ценителей спиртных
Стаут – это темный сорт эля, который изготавливается с использованием жженого солода. Как правило,
IPA — самый популярный стиль крафтового пива в США. Журнал Lucky Peach создал гайд,